Hydrogen fuel is emerging as a key player in the rapidly growing clean energy market. However, hydrogen can contain impurities, introduced during production, purification and along the hydrogen supply chain, which limit the efficiency of fuel cells and contribute to pollution. With pressure on companies involved in the supply chain to maintain the purity of the hydrogen, they need to be able to detect even the tiniest amounts of impurities. An analytical technique called thermal desorption–gas chromatography-mass spectrometry can identify and quantify a wide range of compounds down to parts per trillion levels in accordance with regulations. Researchers will then be able to pinpoint the exact cause of fuel cell performance issues and find ways to avoid impurities entering the hydrogen supply or removing them.
Why hydrogen fuel is important
Scientists have shown that to avert the worst impacts of climate change and preserve a liveable planet, the global temperature increase needs to be limited to 1.5°C above pre-industrial levels.1 To keep global warming to no more than 1.5°C – as called for in the Paris Agreement2 – emissions need to be reduced by 45% by 2030 and reach net zero (cutting greenhouse gas emissions to as close to zero as possible) by 2050.
Hydrogen fuel has been identified as an alternative energy source to address the challenge of reaching net zero emissions. According to the Hydrogen Council (a global initiative that provides guidance on accelerating the deployment of hydrogen solutions), hydrogen will be able to supply more than 20% of the global energy demand by 2050.3
One example of the use of hydrogen is in hydrogen-fuelled vehicles. An electric motor powers these, but instead of plugging them in to charge like electric vehicles, they produce their own electricity inside an onboard fuel cell. Inside the cell, hydrogen reacts with oxygen in a process called reverse electrolysis. The reaction takes place on a catalyst. The hydrogen comes from one or more tanks built into the vehicle, filled at hydrogen refuelling stations, while the oxygen comes from the ambient air. The only products of the reaction are the electrical energy used to power the vehicle, heat and water. The water is emitted as water vapour, making hydrogen-powered cars locally emission-free.
Contaminants are a problem
However, the widespread adoption of hydrogen as a fuel could be challenged by the presence of contaminants that enter the fuel during the production or purification stages or elsewhere along the supply chain. Any impurities may result in substantial degradation of the fuel cell, even at very low concentrations (parts per billion).
Hydrogen impurities include volatile organic compounds (VOCs) that interfere with performance, accelerate degradation and sometimes cause permanent damage to fuel cell components:
- Hydrocarbons adsorb onto the catalyst’s surface, reducing the surface area, and impeding its ability to work properly. Hydrocarbons break down to release carbon monoxide, which also adsorbs onto the catalyst’s surface.
- Sulfur compounds (mainly hydrogen sulfide) bond to the catalyst, deactivating it permanently.
- Halogenated compounds can cause irreversible performance degradation of the fuel cell.
- Formaldehyde and other aldehyde species such as acetaldehyde are very reactive and can readily decompose to release hydrogen and carbon monoxide, both of which degrade platinum catalysts.
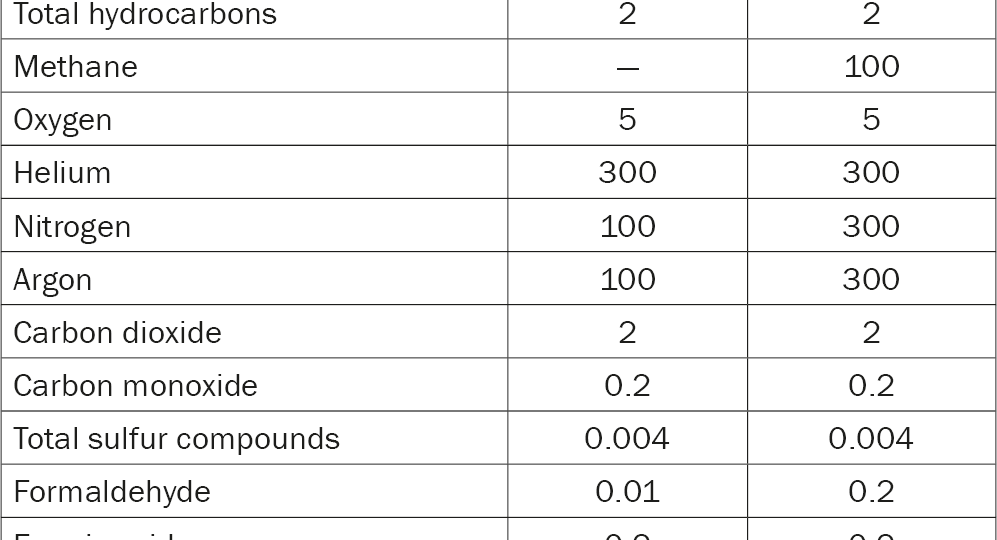
International hydrogen fuel quality standards (ISO 14687, EN 17124, ISO 21087, GB/T 37244, ASTM D7892 and SAE J27194–9) specify maximum concentrations of contaminants for commercial fuel cells. Hydrogen producers and suppliers must safeguard hydrogen quality in accordance with these standards by analysing samples for all, or a subset of the contaminants. Four key standards and their contaminant limit levels are listed in Table 1.
Table 1: Hydrogen purity standards and associated limit levels.
How can such low levels of contaminants be detected and measured?
Trace levels of hydrocarbons, sulfur-containing compounds, halogenated compounds and aldehydes can be detected by thermal desorption–gas chromatography-mass spectrometry/other types of detection instruments. Thermal desorption instruments prepare samples for analysis by increasing the concentration of a sample prior to injection into the gas chromatograph. This is called preconcentration. The gas chromatograph then separates the compounds, and the mass spectrometer detects them, producing a chromatogram from which analysts can see which volatile organic compounds are in the sample.
There are different ways to prepare samples for hydrogen fuels depending on sample location, stage of the supply chain and priority impurities for measurement. Some analysts will want to see what is in the entire sample – this approach is called a non-targeted approach. The targeted approach involves looking for a specific compound or group of compounds and this would be useful for quality control or for fuel cell research and development.
There are two ways to take samples for hydrogen fuels:
- On-line monitoring of gas streams: Automated, scheduled sampling and analysis from a hydrogen gas stream provides a regular measure of hydrogen supply purity at source and along the supply chain. The hydrogen gas stream is directed into the thermal desorption instrument where the volatile impurities are preconcentrated before injection to the gas chromatograph. Results can be obtained within minutes of taking the samples.
- Off-line sampling: Where it is not practical or cost-effective to install a full analytical setup for every sampling point, a special bag, canister or sorbent tube (a small tube containing a sorbent material that traps the volatile compounds of interest), can be used to collect a sample, which is then returned to a laboratory for analysis.
There are also different detectors for detecting specific compounds. A mass spectrometer is used for the non-targeted profiling of an entire sample. To target specific chemicals of interest, other detectors can be used. Flame ionisation detectors enable analysts to identify halogenated and hydrocarbon compounds. Sulfur chemiluminescence detectors target sulfur-containing compounds. Electron capture detectors can be used to target halogenated compounds. The detectors can be combined on the same gas chromatograph. Figure 1 shows the sampling methods that can be used for each compound group.
Figure 1: Different types of sampling and analysis methods for hydrogen fuel impurities, highlighting the complementary nature of the different sampling methods.
Another point to mention is that instruments should be certified as safe to work with hydrogen. Since 2021, a range of Markes International’s thermal desorption instruments has been multi-gas-enabled. ‘Multi-Gas’ is an award-winning technology that enables the user to choose one of three carrier gases (which are used to transfer volatile compounds through the analytical system) – helium, nitrogen or hydrogen. Each multi-gas instrument has been independently evaluated and certified for hydrogen carrier and sample gas so that the full analytical workflow can be safely configured with hydrogen.
Putting the method into practice
A multi-gas-enabled UNITY–CIA Advantage-xr™ system can be used to sample from on-line gas streams and off-line cylinders and bags. A water removal system – Kori-xr™ – can be added for humid samples. Water in samples is a problem because it can mask some compounds in the analytical data. Also, an ULTRA-xr™ autosampler was added. This enables unattended sampling of up to 100 sorbent tubes in a single sequence.
The system was used with a gas chromatograph and mass spectrometer to analyse a high-volume sample of a 10-ppb (parts per billion) standard containing 60 compounds of interest and 50 ppm (parts per million) of water (ten times the maximum water content listed in ISO 14687).10 All 60 compounds were identifiable on the resulting chromatogram (Figure 2) and the water was successfully removed (it would have appeared as a large peak on the chromatogram otherwise).
Figure 2: Total ion chromatogram showing the peaks of interest for the 60 compounds of interest, produced from a high-volume sample of 10-ppb standard in humid hydrogen gas.
Excellent limits of detection (smallest concentration of a measurand that can be reliably measured by an analytical procedure) values were achieved with an average of 16 ppt (parts per trillion) across all 60 compounds, with the highest being 88 ppt for isopropanol and the lowest being 4 ppt for chlorodibromomethane. The values for all compounds are significantly lower than required by standard methods, for example, ISO 14687, which gives a maximum allowable concentration of 2000 ppb for total hydrocarbons, 4 ppb for total sulfur compounds and 50 ppb for total halocarbons. An equally impressive limit of quantitation values was achieved with an average of 54 ppt across all 60 compounds, with the highest being 292 ppt for isopropanol and the lowest being 15 ppt for chlorodibromomethane and tetrachloroethene. The limit of quantitation is the lowest concentration that can be determined with acceptable precision.
Compound-specific detector
Measuring total sulfur content is a priority in hydrogen fuel impurity analysis because it is so destructive to the fuel cell, so a sulfur chemiluminescence detector was employed instead of the mass spectrometer in conjunction with the same thermal desorption and gas chromatography setup. The detector is designed to detect sulfur compounds, reducing potential analytical interference from other impurities such as carbon dioxide, allowing for larger sample volumes and enhancing sensitivity for sulfur-containing compounds. Using sulfur chemiluminescence detection with preconcentration on a thermal desorption instrument, it is possible to detect exceptionally low levels of sulfur compounds. The results in Figure 3 show reliable detection and high sensitivity, even at 20 ppt, which is far below the required detection limit for sulfur compounds.
Figure 3: Thermal desorption–gas chromatography–sulfur chemiluminescence data showing six replicate analyses of priority sulfur-containing compounds at 50 ppt.
Conclusions
Thermal desorption with gas chromatography-mass spectrometry is a powerful technique that exceeds the requirements of quality standards such as ISO 14687, EN 17124, SAE J2719 and ASTM D7892 for the analysis of VOC hydrogen fuel impurities. The results will give researchers the data they need to be able to find the causes of fuel cell degradation and find ways to remove them.
References
- https://www.un.org/en/climatechange/net-zero-coalition.
- https://www.un.org/en/climatechange/paris-agreement.
- https://hydrogencouncil.com/wp-content/uploads/2021/11/Hydrogen-for-Net-Zero.pdf.
- ISO 14687:2019, Hydrogen fuel quality – product specification, International Organization for Standardization, Geneva, Switzerland, https://www.iso. org/standard/69539.html.
- ISO 21087, International standard, Gas analysis – Analytical methods for hydrogen fuel – Proton exchange membrane (PEM) fuel cell applications for road vehicles, https://www.iso.org/standard/69909.html.
- GB/T 37244, Chinese standard, Fuel specification for proton exchange membrane fuel cell vehicles – Hydrogen, https://www.standardsofchina.com/standard/GBT37244-2018.
- EN 17124:2022, Hydrogen fuel. Product specification and quality assurance, Proton exchange membrane (PEM) fuel cell applications for road vehicles, European Committee on Standardisation, Bruxelles (2022), https://www. en-standard.eu/une-en-17124-2022-hydrogen-fuel-product-specification-and-quality-assurance-for-hydrogen-refuelling-points-dispensing-gaseous-hydrogen-proton-exchange-membrane-pem-fuel-cell-applications-for-vehicles/.
- ASTM D7892, Standard test method for determination of total organic halides, total non-methane hydrocarbons, and formaldehyde in hydrogen fuel by gas chromatography/mass spectrometry, https://www. en-standard.eu/astm-d7892-22-standard-test-method-for-determination-of-total-organic-halides-total-non-methane-hydrocarbons-and-formaldehyde-in-hydrogen-fuel-by-gas-chromatography-mass-spectrometry/.
- SAE J2719: Hydrogen fuel quality for fuel cell vehicles, https://www.sae.org/standards/content/j2719_201109/.
- Markes International Application Note 165: Optimised hydrogen fuel impurity analysis: identification, measurement and characterisation of volatile organic compounds by TD–GC–MS/SCD.




